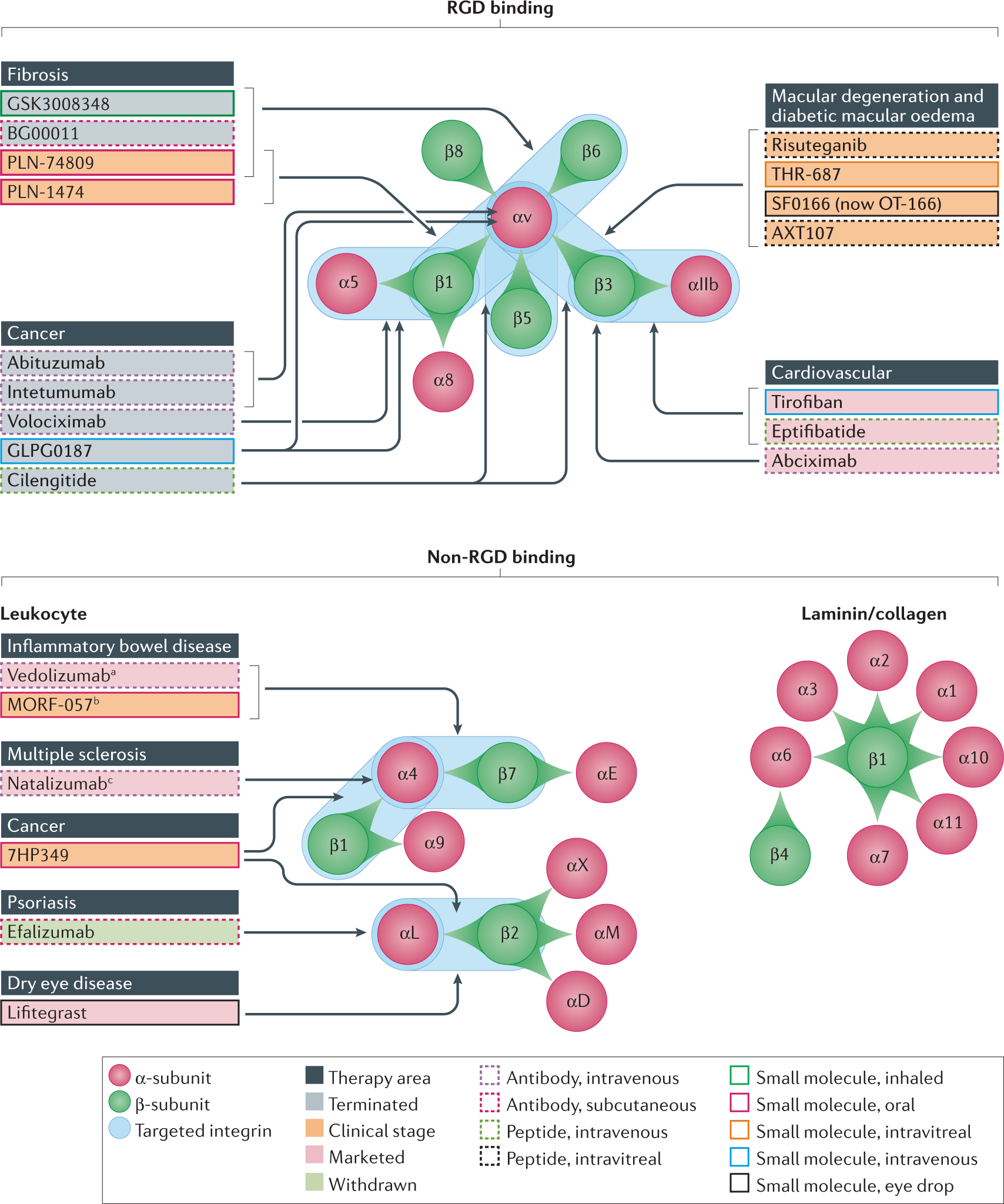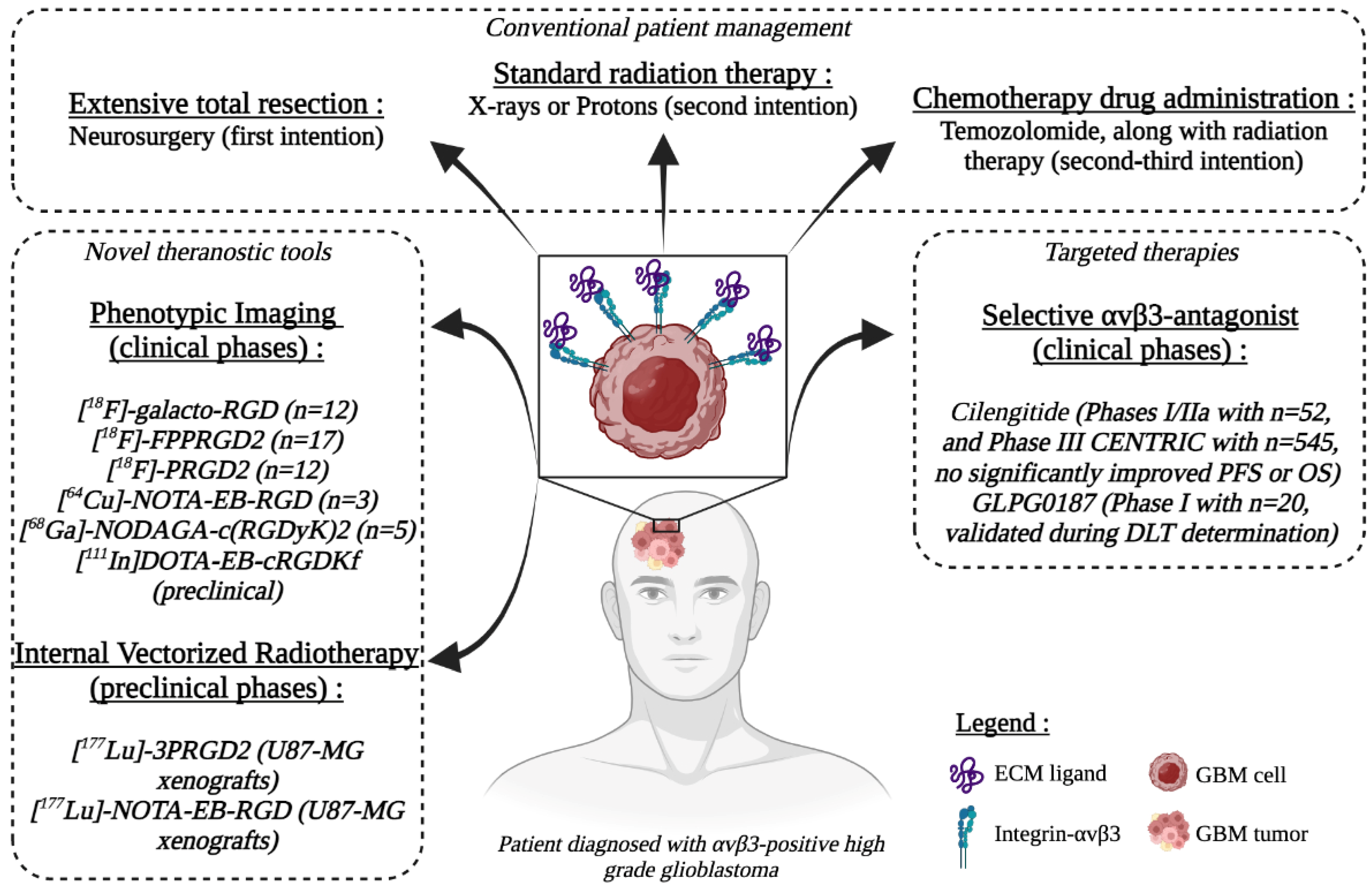
Pharmaceutics | Free Full-Text | Integrin-αvβ3 as a Therapeutic Target in Glioblastoma: Back to the Future?
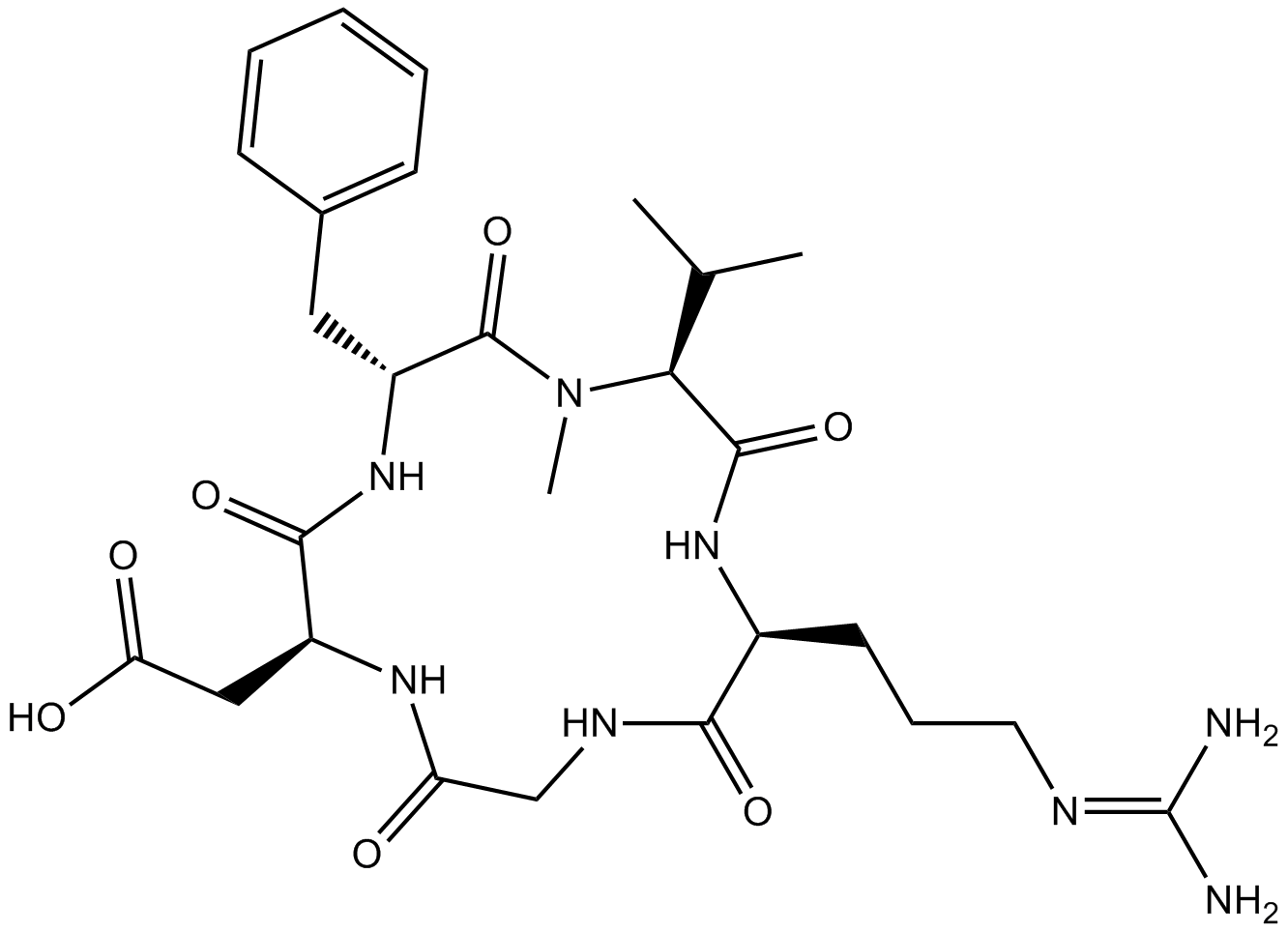
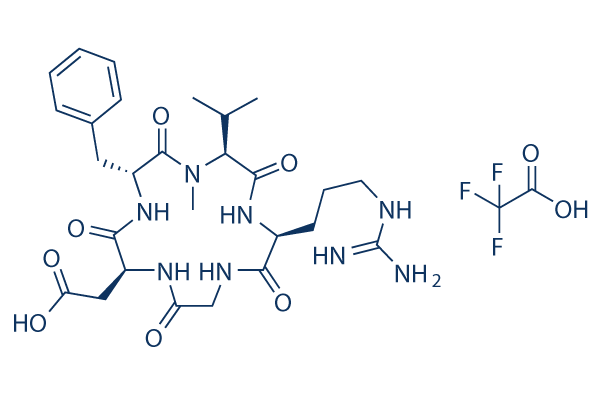
Cilengitide | CAS:188968-51-6 | Integrin inhibitor for αvβ3 and αvβ5 | High Purity | Manufacturer BioCrick

Does Valproic Acid or Levetiracetam Improve Survival in Glioblastoma? A Pooled Analysis of Prospective Clinical Trials in Newly Diagnosed Glioblastoma | Journal of Clinical Oncology

Full article: Cilengitide, an αvβ3-integrin inhibitor, enhances the efficacy of anti-programmed cell death-1 therapy in a murine melanoma model
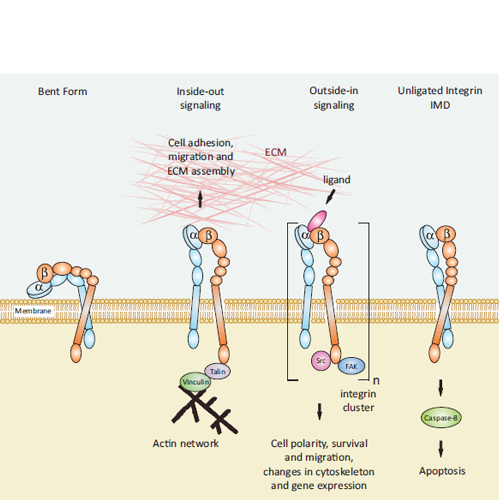
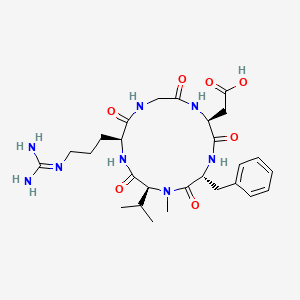
CIPSM - Cilengitide: The First Anti-Angiogenic Small Molecule Drug Candidate. Design, Synthesis and Clinical Evaluation

Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial - The Lancet Oncology
![PDF] Integrin inhibitor cilengitide for the treatment of glioblastoma: a brief overview of current clinical results. | Semantic Scholar PDF] Integrin inhibitor cilengitide for the treatment of glioblastoma: a brief overview of current clinical results. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/70bc1a06a730e1f512f80dac37d33d6c52a1670a/5-TableI-1.png)
PDF] Integrin inhibitor cilengitide for the treatment of glioblastoma: a brief overview of current clinical results. | Semantic Scholar
NEWS RELEASE MDxHealth and Merck KGaA extend Agreement for use of MGMT Assay in Brain Cancer Clinical Trials

Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03-02, a phase II trial with measures of treatment delivery | SpringerLink

Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial - The Lancet Oncology

Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. - Abstract - Europe PMC
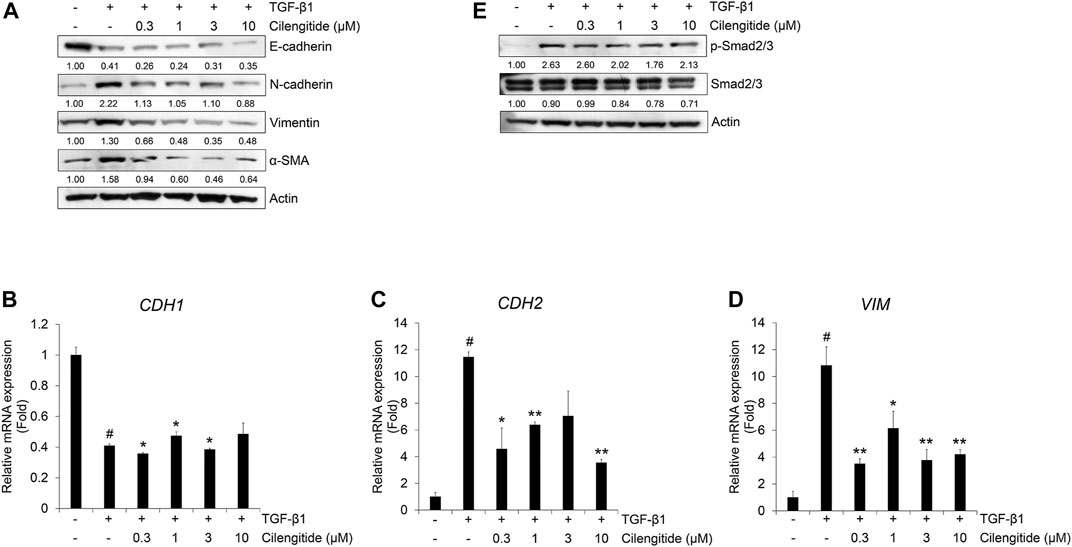
Frontiers | Cyclic RGD Pentapeptide Cilengitide Enhances Efficacy of Gefitinib on TGF-β1-Induced Epithelial-to-Mesenchymal Transition and Invasion in Human Non-Small Cell Lung Cancer Cells
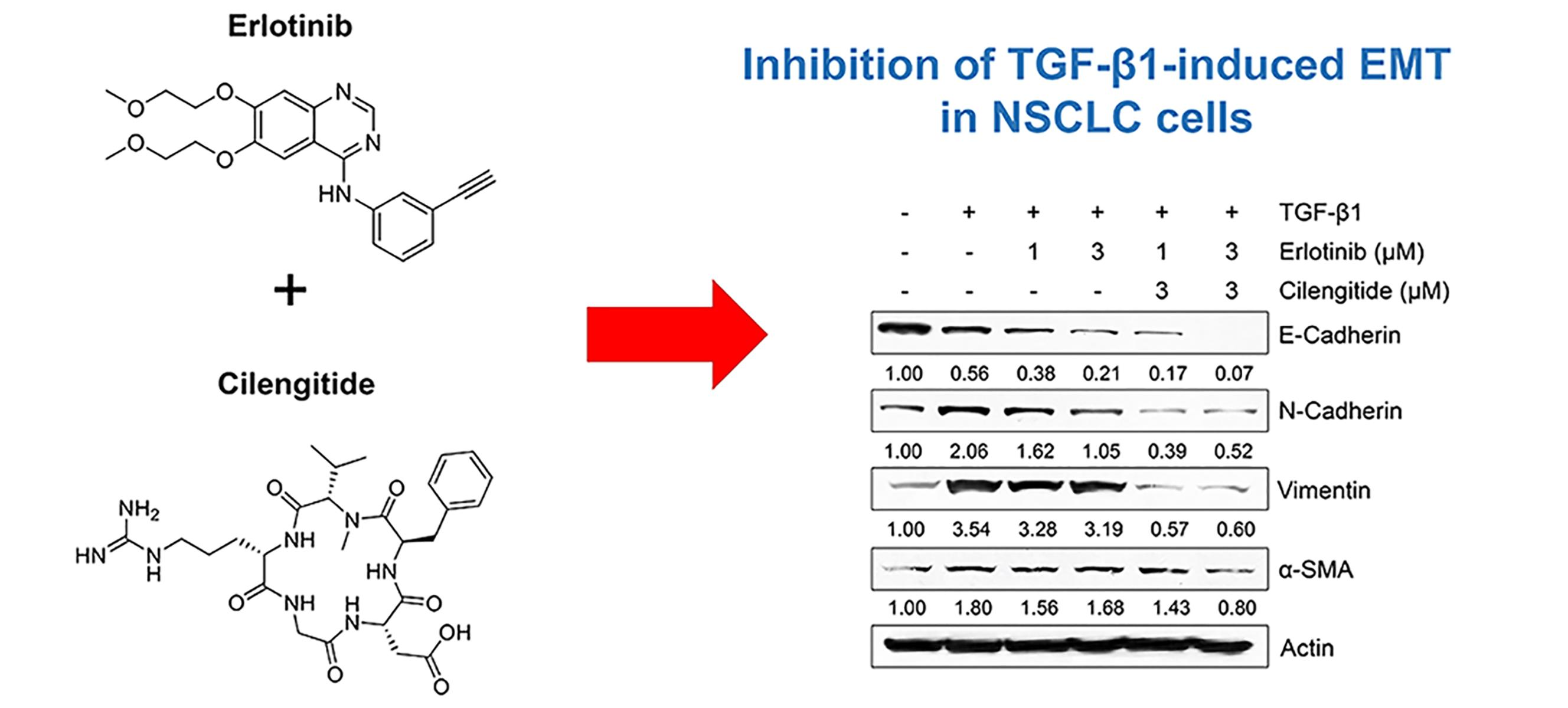
IJMS | Free Full-Text | Combination Effect of Cilengitide with Erlotinib on TGF-β1-Induced Epithelial-to-Mesenchymal Transition in Human Non-Small Cell Lung Cancer Cells