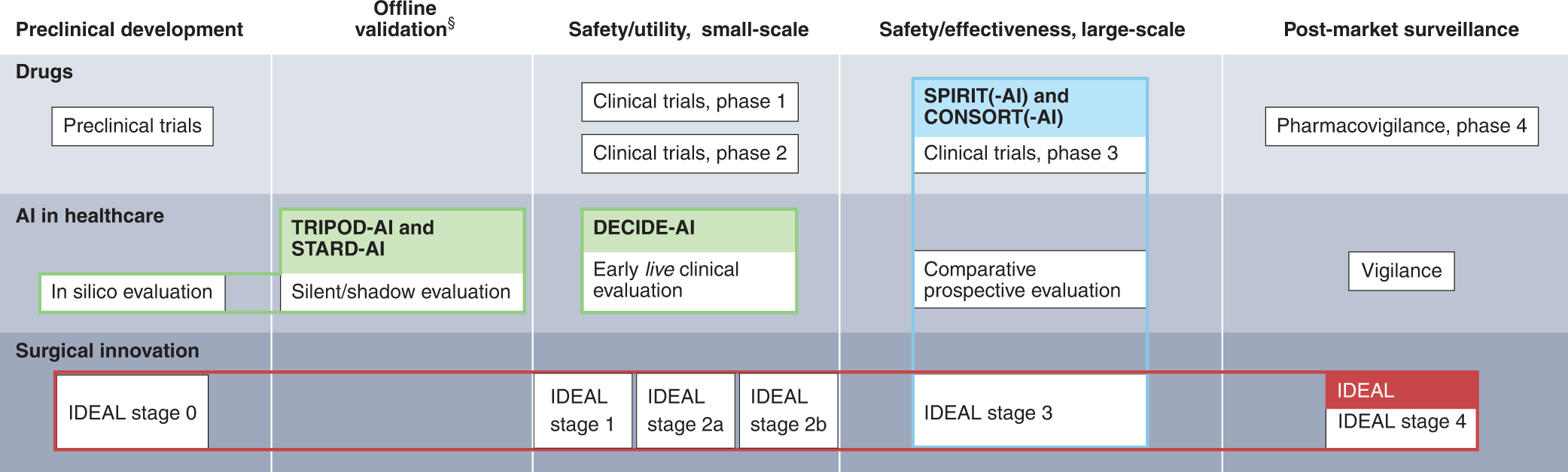
Guidance for Deploying Medical Image-based Software – including Algorithms – in the Clinical Setting

PDF) Clinical investigation plan for the use of interactive binocular treatment (I-BiT) for the management of anisometropic, strabismic and mixed amblyopia in children aged 3.5–12 years: a randomised controlled trial
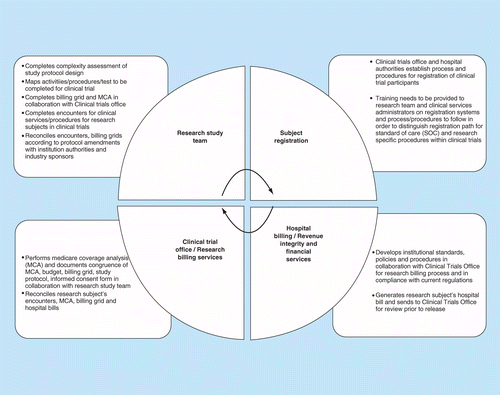
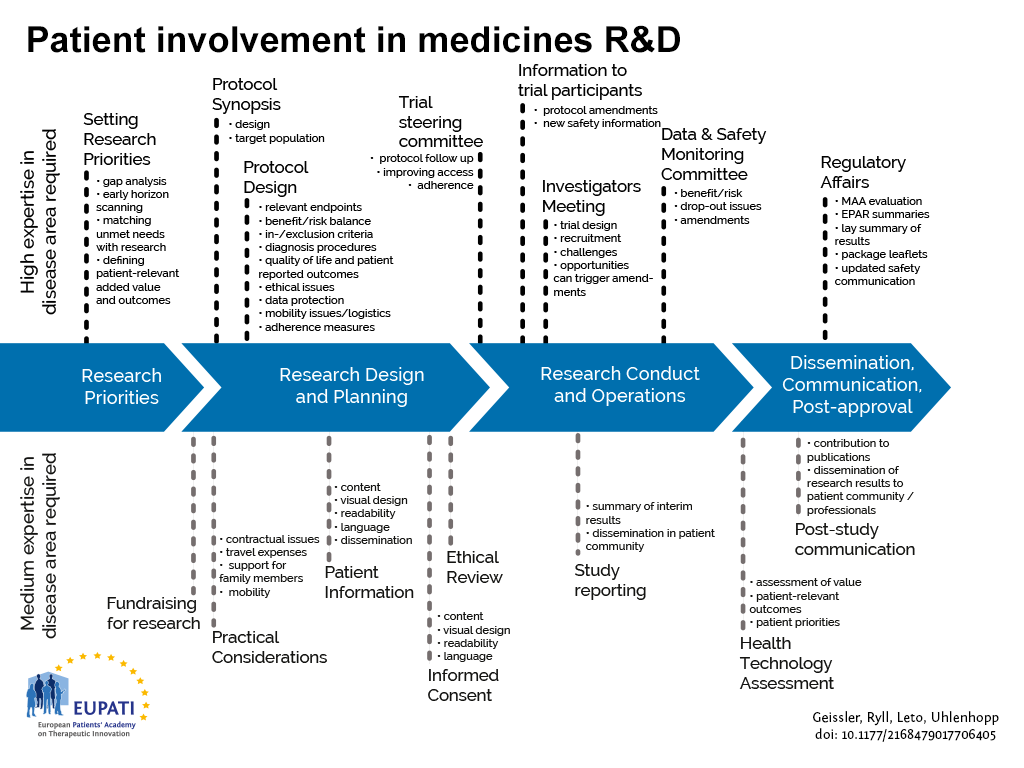
Optimization of protocol design: a path to efficient, lower cost clinical trial execution | Future Science OA