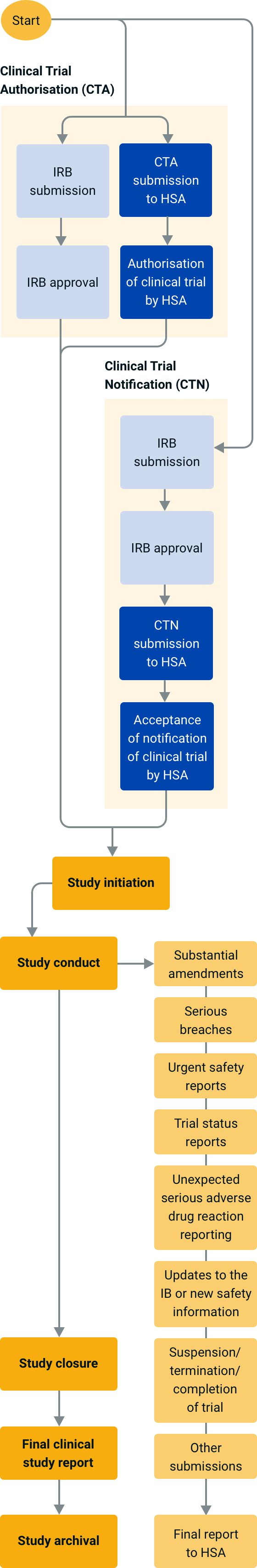
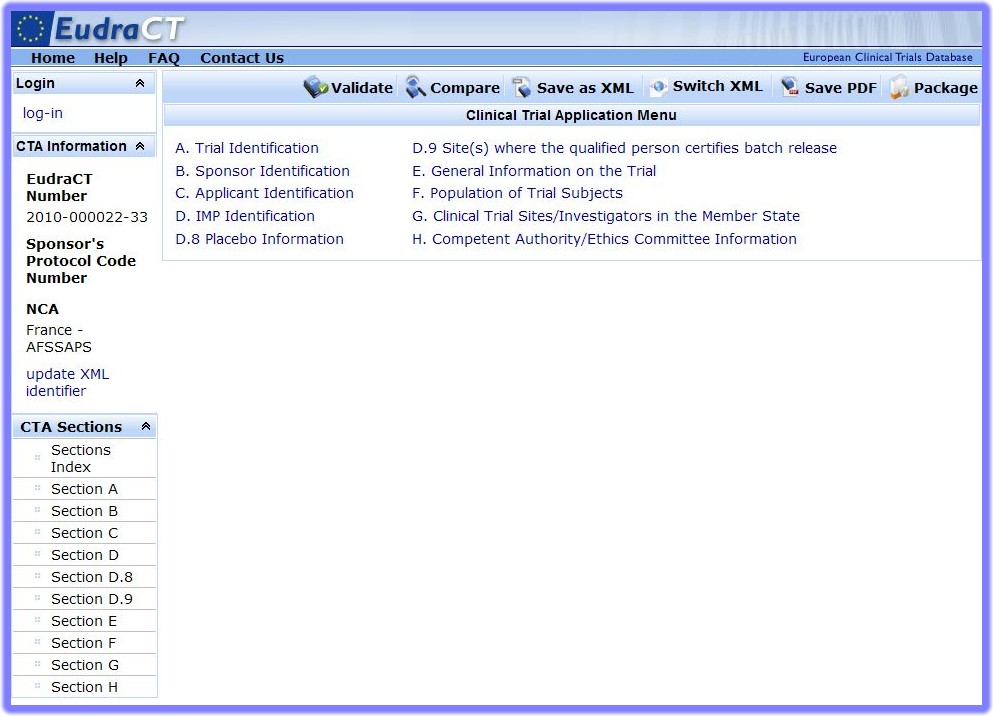
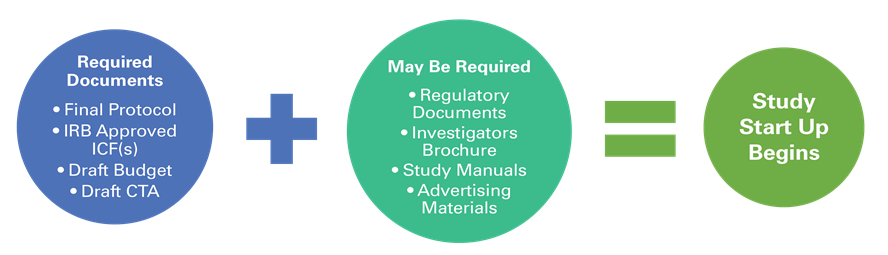
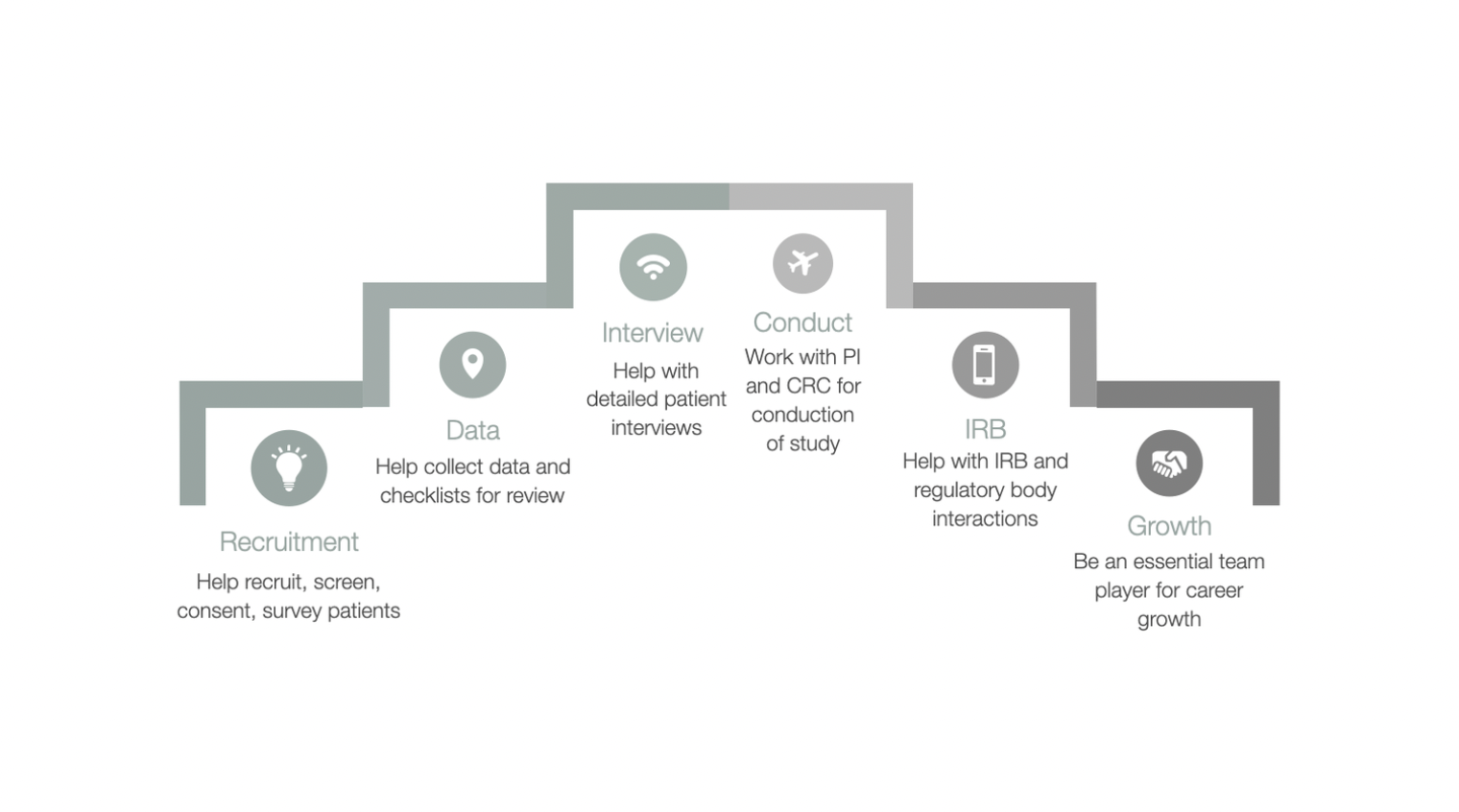
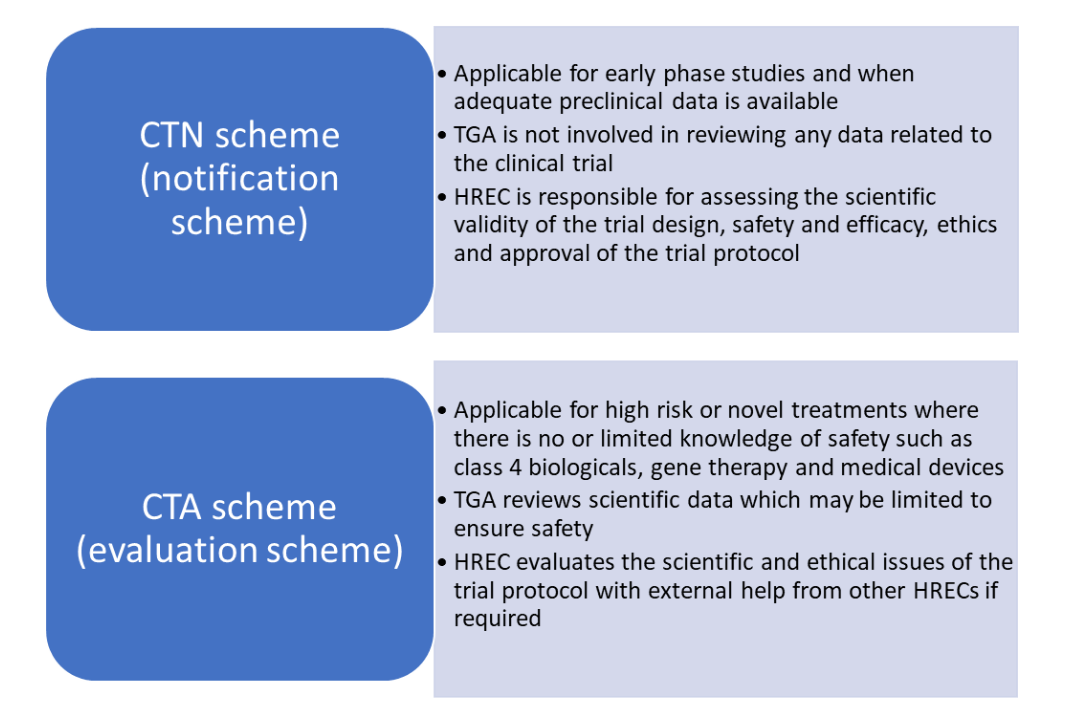
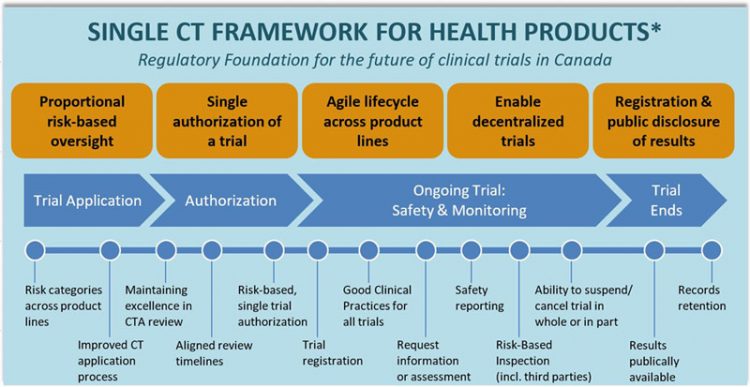
Regulatory Affairs 101: Introduction to Investigational New Drug Applications and Clinical Trial Applications - Chiodin - 2019 - Clinical and Translational Science - Wiley Online Library
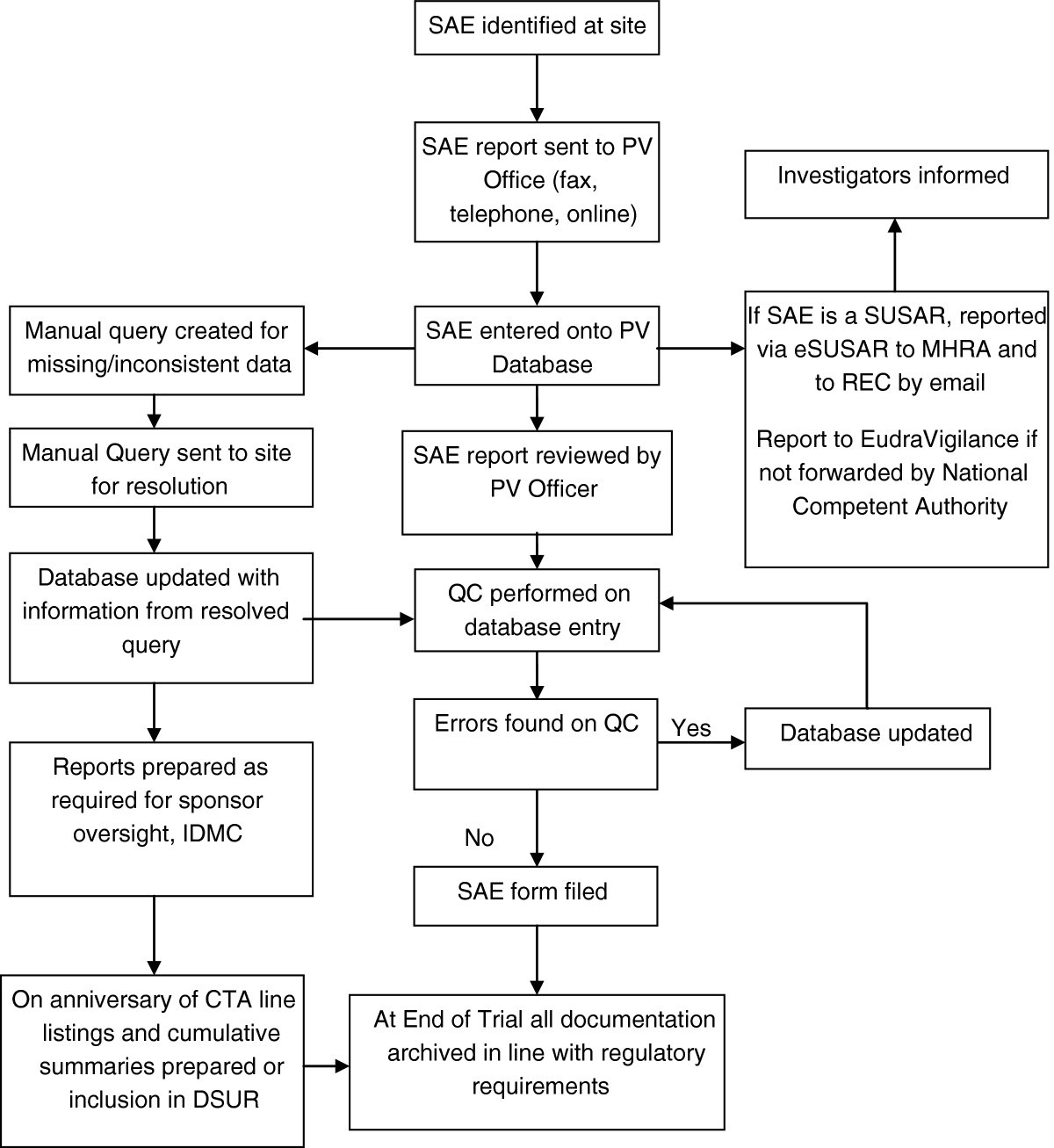
Implementing a centralised pharmacovigilance service in a non-commercial setting in the United Kingdom | Trials | Full Text